Full
FullGuide »

January 2025
Pea & Bean Seed Quality
The achievement of an optimum plant population will provide the greatest economic yield while taking into account the high cost of seed and it is necessary to calculate the seed rate required to obtain this. It is also essential to use only seed of high quality.
The quality of a seed lot is determined by germination capacity, vigour and the absence of seed-borne pests, diseases and disorders. The information given here will help the grower to recognise the value of high-quality seed and PGRO can further assist in this choice.
GERMINATION CAPACITY, PURITY AND THOUSAND GRAIN WEIGHT
These can easily be checked using routine tests. They provide important information needed to calculate the seed rate. The optimum population depends on seed cost and return of produce per hectare. Adjustments should be made accordingly. Recommended target plant density for vining peas is 100 plants/ m2, for green beans 35 to 40 plants/ m2, for combining peas between 65 and 100 plants/ m2, for winter beans between 18 and 30 plants/ m2 and for spring beans between 40 and 55 plants/ m2. Further information for pulses is available in the PGRO Agronomy Guide. Some varieties are sold in units which are pre-calculated weights for a given area. However, for other seed, the seed rate can be calculated from the following formula:

Use the seed rate formula and adjust for expected field losses to achieve the most profitable population. Expected field losses are 15% when sown very early (February) and 10% when sown early (March). Germination capacity can be checked using routine tests available at PGRO.
SEED-BORNE DISEASES
Pea seed can be infected with Ascochyta spp., comprising Ascochyta pisi, Didymella pinodella and Didymella pinodes, and broad and field bean seed with Ascochyta fabae. There is a fungicidal seed treatment (Prepper – fludioxonil) available to give partial control of Ascochyta pisi in peas. Ascochyta
fabae can be very damaging in field beans and it is strongly recommended that seed is tested. It is advised that Basic seed of field beans should not contain more than 0.2% infection, C1 seed no more than 0.4% and C2 seed no more than 1%. Pea seed should contain no more than 5% infection with Ascochyta diseases.
Green beans can be infected with halo blight (Pseudomonas syringae pv. phaseolicola), for which no treatment is available. Pea bacterial blight (Pseudomonas syringae pv. pisi) has been a problem in winter peas historically, but winter peas are generally no longer grown. Occasional problems have occurred in spring sown combining or vining peas. There is no chemical control and repeated multiplication of infected seed should be avoided.
Pea seed-borne mosaic virus occurs in vining and combining pea seed. The only means of control is by using healthy seed and a test is available from PGRO.
Two seed-borne diseases of field beans, broad bean stain virus and broad bean true mosaic virus, have occasionally caused some yield loss. The viruses are weevil-transmitted, but levels of seed-borne infection are seldom of significance.
DISORDERS
Peas or beans produced in areas where the soil is above pH 6.8 are likely to be deficient in manganese. The resulting seed may then display symptoms known as marsh spot, a brown necrotic area in the centre of the surface of the cotyledons. Severely affected seeds produce abnormally developed seedlings (see PGRO Technical Update 01).
Seed of peas and beans, especially green beans, is very fragile owing to its very dry condition at harvest, and it is vulnerable to damage during handling. The embryonic plumule can easily become broken within the cotyledons, and on germination the resulting seedling then shows the "baldhead" symptom. Although the amount of baldhead in a sample may be recorded in a germination test, posttest handling and damaging seed drill mechanisms can result in an even greater proportion of this type of loss. Handle seed, particularly green bean seed, with great care.
Seed coat cracks are an important cause of low vigour in vining peas. Care should be taken when handling dry seed and especially when harvesting vining pea seed crops.
PESTS
Broad and field beans may become infested with stem and bulb nematodes (Ditylenchus gigas and Ditylenchus dipsaci) which travel through the plant and into the seed, where they can remain viable underneath the testa in a dried condition for several months. When infested seed is drilled, the resulting plant may become heavily parasitised and the soil in which it is sown also becomes infested, providing a source of infestation for future susceptible crops. Seed can be tested by PGRO and where an infestation is present then it is advisable not to use the seed (see PGRO Agronomy Guide). PGRO can also carry out a soil test to determine presence, and a test to determine species is also available.
Holes in bean seed are caused by bean seed beetles (Bruchids – see PGRO Technical Update 10) which emerge from the seed either just before or just after dry harvest. The majority of damaged beans germinate normally, and a standard germination test will determine any potential problem.
SEED VIGOUR
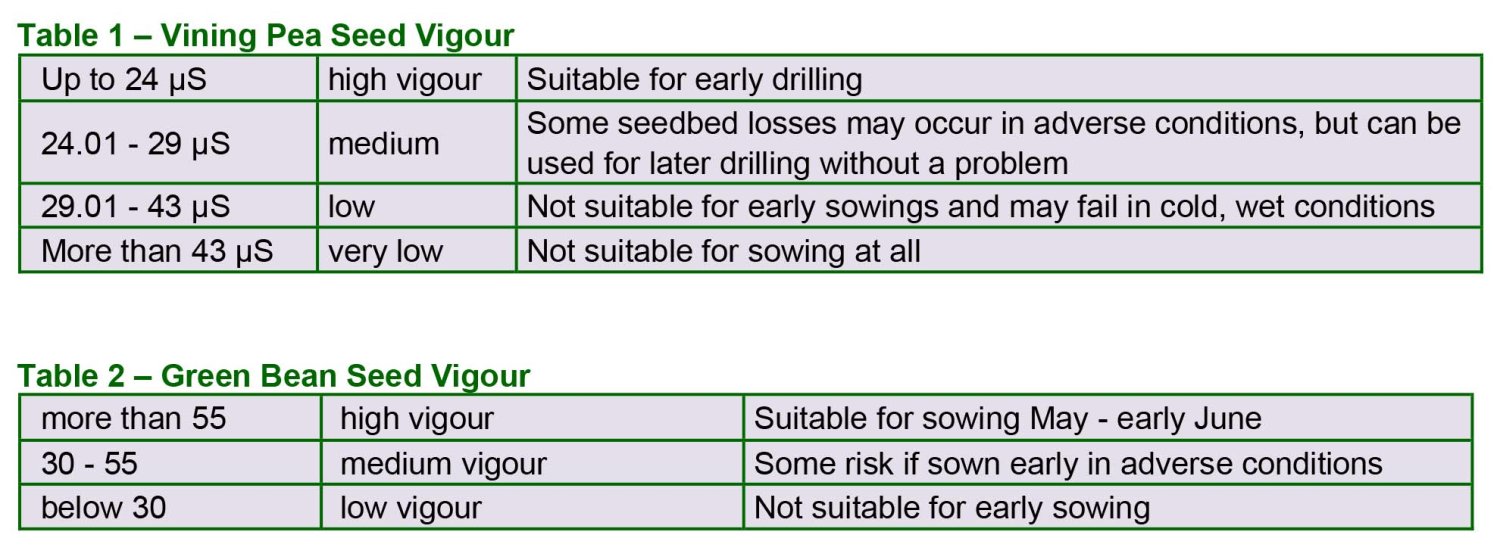
The result of the germination test is not always a reliable guide to seedling establishment in the field. Vining peas, for example, are prone to emergence failure when sown in adverse soil and weather conditions. The ability of the seed to survive and emerge in field conditions is known as "vigour". Vining pea seed can be tested for vigour using the electrical conductivity method (see PGRO Technical Update 35). The result obtained is given in units of microsiemens/g, and any value can be related to expected field performance using Table 1 below. Vining pea seed may also be affected by hollow heart. PGRO research over several years has shown that seed with a high level of hollow heart should not be sown in conditions where seed is likely to be under stress.
Combining peas, marrowfats or round-seeded types seldom suffer from seedbed losses due to low vigour and therefore the test is of limited value for these types of peas.
In green beans a vigour test has been developed based on the number of seeds which show complete cotyledon staining by tetrazolium chloride. The results of the test are expressed as a percentage and the expected field performance can be assessed using Table 2.
SEED TESTING SERVICE
PGRO offer to members a full range of tests including germination, disease and vigour assessments. Full details can be found on the PGRO web site www.pgro.org.