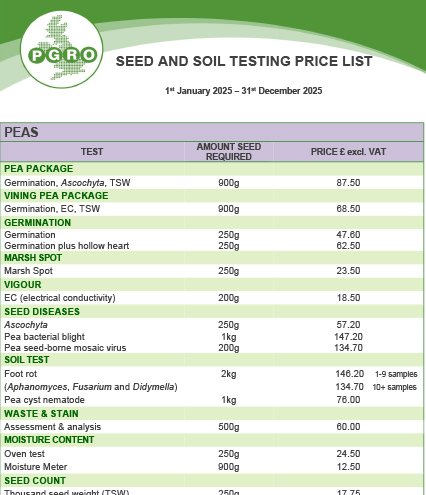
Soil Testing
A full range of seed tests for peas, beans and other crops is available.
Foot rot diseases in peas are caused by a complex of soil borne pathogens. The most common pathogens are Fusarium solani, Didymella pinodella and Aphanomyces euteiches. These pathogens can occur individually or in combination, and yield losses can be severe.
Development of foot rot diseases is encouraged by poor soil structure, compaction and water logging. All three pathogens produce long-lasting resting spores which survive in soils for more than ten years.
Frequent legume cropping leads to the build-up of pathogen levels in soils. Work has shown that some degree of yield loss can even occur where relatively low levels of pathogens are present, and the above ground symptoms of the disease may not be obvious. Soils tests are available at PGRO to test levels of the above mentioned foot rot pathogens in soils.
These levels are linked to risks of foot rot development when conditions are favourable for disease development. A foot rot score is assigned to the individual pathogens and an overall risk score for the soil which indicates the risk level of disease development is provided. Test results allow growers to avoid planting peas in fields which show a high risk of foot rot pathogen presence.
Soil samples should be collected at the latest in autumn before pea planting. Test results will also be valid for soils tested up to 2 years in advance of planting peas due to the long lasting nature of the pathogen spores. Occasionally, soil samples may have to be taken from fields containing standing crops, eg. sugar beet. In these cases, soil from the surface should be discarded as some residual herbicides can affect the result. Test results will be provided 4 weeks after receiving of soils.
Molecular soil testing services:
PGRO offer rapid molecular testing services for the detection and identification and risk level evaluation of soil borne:
- Brassica club root (Plasmodiophora brassicae)
- Stem and bulb nematodes (Ditylenchus dipsaci and Ditylenchus gigas)
Brassica clubroot:
Clubroot (Plasmodiophora brassicae) is a disease affecting vegetable Brassicas and oil seed rape. Typical symptoms are galls formed on roots affecting water and nutrient uptake. Plants are stunted, often wilt in dry conditions or can die off completely. The pathogen multiplies in the galls and long-lasting resting spores are released to the soil. These spores can survive in soils for more than 15 years. In oil seed rape, resistant varieties exist but resistance has broken down in large areas of the UK and cannot be replied upon any longer.
Lengthening rotations is the only mean to control clubroot sustainably and information on pathogen levels in soils aid rotational planning. PGRO is offering a test to quantify clubroot spore numbers in soils. The test had originally been developed by the University of Worcester who provided a risk table.
|
Spore number per g soil |
Risk categorisation |
Estimated infection level |
|
<5,000 |
Low |
Unlikely to get more than 33% infection |
|
5,000-100,000 |
Medium |
Likely to get between 33% and 66% infection |
|
>100,000 |
High |
Likely to get more than 66% infection |
A 1 kg soil sample is required for testing. We recommend to sample to a depth of 20 cm at a minimum of 25 locations per field to get a representative sample. Test results will be provided within 10 days of receiving the soil sample.
Nematodes:
Stem and bulb nematodes, Ditylenchus dipsaci and Ditylenchus gigas, are microscopic worms of great agricultural importance, affecting a variety of crops. Ditylenchus dipsaci is known to infect over 450 crops and weeds, including Allium species, oats, daffodils, Phaseolus beans, field beans and peas. Ditylenchus gigas is mainly found in spring and winter field beans, as well as several weeds, and has the greater impact on yield in field beans.
Both nematode species are seed- and soil-borne, hence infested seeds and bulbs or contaminated soils and equipment are potential sources of infestation. The damage caused by this pest can be devastating since nematodes reproduce rapidly, potentially leading to significant yield loss.
Stem and bulb nematodes are resistant organisms enduring sub-zero winter temperatures, summer soil temperatures of 55°C, and can survive in a desiccated form for years or decades in plant debris, seed, and soil.
Prevention and control of this pest is best achieved by testing seeds and bulbs for the presence of nematodes, crop rotation and equipment disinfection between fields. A soil test determining nematode presence in soil can aid rotational planning and reduce risks of infection.
PGRO is offering a new soil test for the detection of stem and bulb nematodes based on molecular diagnostic methodologies. The test determines the presence of the pest, with a detection limit of 10 nematodes/100g soil. Presence of Ditylenchus dipsaci and Ditylenchus gigas is determined separately. To test soil for the presence of nematodes, send a 2 kg soil sample to the PGRO lab. Nematodes move with the water table in the soil profile so best sampling times are in autumn or spring after rainfall. Sampling in dry periods or over winter are not suitable. Test results will be provided within 10 days of receiving the samples.
Risk table for clubroot:
|
Pathogen |
Low risk |
Medium risk |
High risk |
|
Aphanomyces euteiches |
0 - 1.8 |
1.81 - 3.2 |
3.21 - 5 |
|
Fusarium solani |
0 - 1 |
1.01 - 1.99 |
>2 |
|
Didymella pinodella |
0 - 1 |
1.01 - 1.99 |
>2 |